About CIDP
Chronic inflammatory demyelinating polyneuropathy, or CIDP, is a disorder where antibodies in the immune system attack healthy tissue, affecting the nerves in the arms and legs.

What is CIDP?
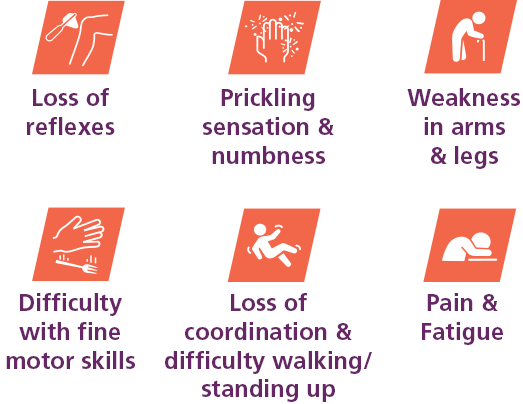
CIDP is a rare medical condition that can be difficult to diagnose. CIDP is most commonly experienced as weakness in the arms and legs, and may be accompanied by a prickling sensation and numbness. Symptoms can happen in waves, coming and going over time, or progress consistently.

How CIDP is treated
Back to topYour care team will work together to treat your CIDP. This may include neurologists, neuromuscular specialists, neurophysiologists, pharmacists, and nurses.
Your care team may choose from these available treatments:
- Intravenous immune globulin (IVIg): Adds new antibodies to the body to help block the attacking antibodies. PANZYGA is an IVIg therapy
- Corticosteroids: Help reduce inflammation and relieve symptoms
- Plasma exchange: Removes attacking antibodies from the body
- Immunosuppressants: Minimize the immune response
Causes of CIDP
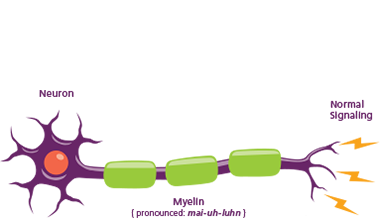
In a healthy body
Neurons deliver messages between the brain and the rest of the body to perform actions like picking up a cup or taking a step. Myelin is a protective layer around a neuron—like insulation around a wire—that helps messages get to their destinations.
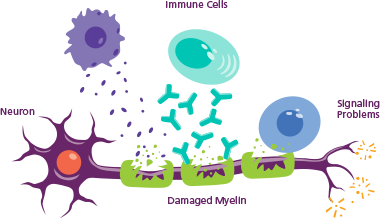
When someone has CIDP
The immune system, which usually recognizes foreign germs in the body and fights them off, starts to think the neurons in a person’s limbs are foreign and attacks. The immune system damages the protective myelin coating, causing the messages neurons deliver to be slowed or lost.
PANZYGA CIDP treatment benefits
Back to topPANZYGA is an intravenous immunoglobulin (IVIg) therapy that is FDA approved for adults with CIDP. A nurse or other member of your care team will give you PANZYGA by intravenous (IV) infusion, which can occur in a hospital, an infusion clinic, or at home.
In a clinical study, PANZYGA improved limb disability and impairment symptoms related to CIDP in adults.
PANZYGA was studied in adult patients with CIDP. Adult patients in the study were divided into groups, each receiving a different dose of PANZYGA. Response to treatment was dependent on dose: the higher the dose, the more people responded.
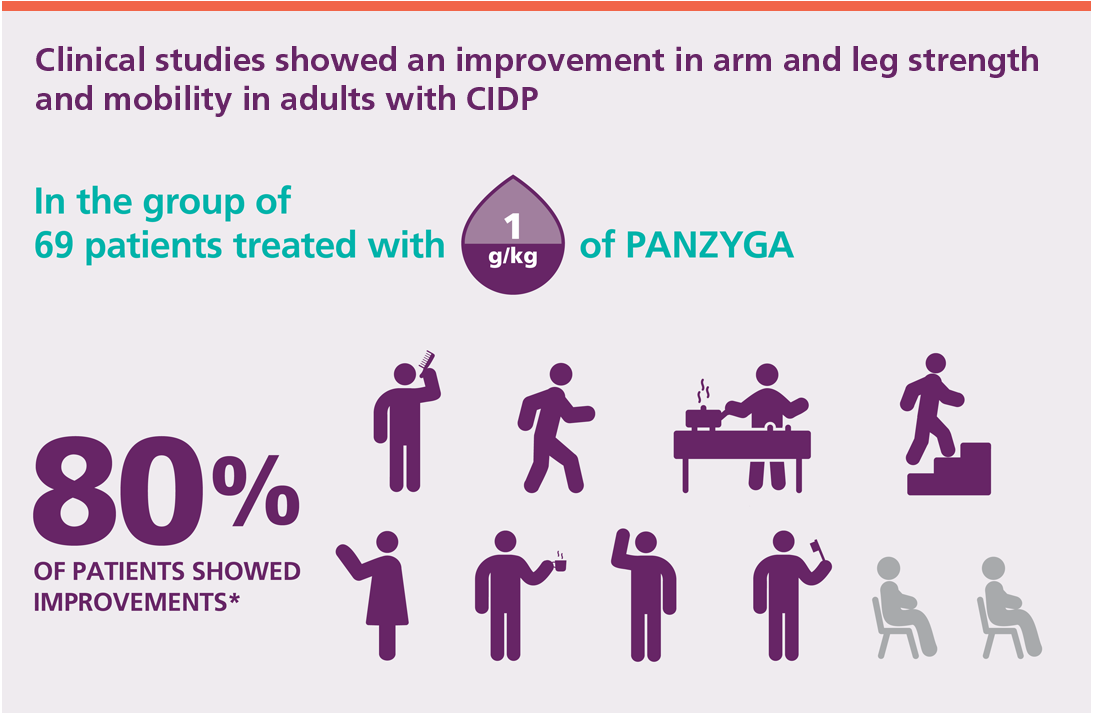

*Response to treatment was an improvement in a patient’s score based on an examination that measures arm and leg mobility.
†Dose-dependent increase in headache was observed in 2 g/kg treatment group.
PANZYGA CIDP side effects
Back to topAdverse reactions reported in >5% of adult patients with CIDP treated with PANZYGA in
a clinical study.
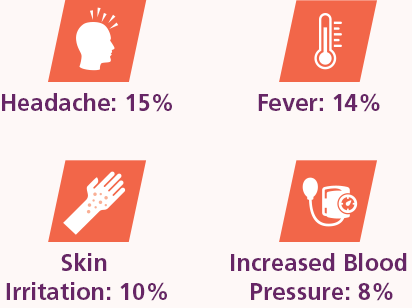
The most common side effects of PANZYGA among patients receiving a 0.5 g/kg, 1 g/kg, or 2 g/kg dose in the CIDP clinical study were headache, fever, skin irritation, and increased blood pressure. Allergic reactions may occur.
Of all side effects:
- 79% were mild
- 18% were moderate
- 3% were severe

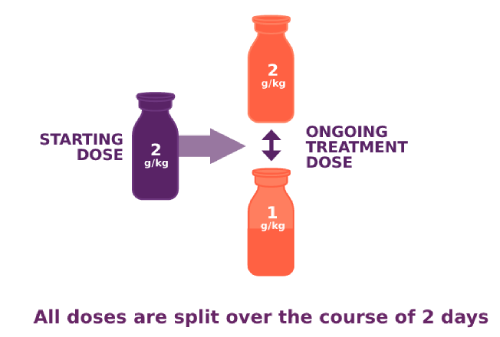
PANZYGA dosing options for adults with CIDP
Back to top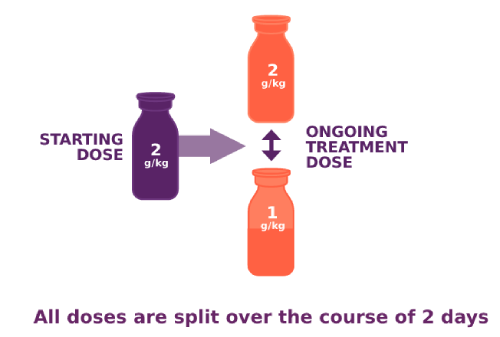
In the clinical study, PANZYGA infusions occurred every 3 weeks. After your starting dose, you and your doctor will work together to determine the best maintenance dose for your ongoing treatments. This dose can be adjusted as needed. PANZYGA was tested at multiple doses in the clinical study, and adult patients saw symptom improvement at both the 1 g/kg and 2 g/kg doses.

CIDP patient foundation
Back to topLearn more about CIDP by visiting the following website:
GBS | CIDP Foundation International
The link above is for your convenience. This website is neither owned nor controlled by Pfizer. Pfizer does not endorse and is not responsible for the content or services of this site.
Stay informed
Back to topLearn more here. When you have questions, it’s best to talk with your healthcare team. Here are some frequently asked questions and answers about PANZYGA treatment to help get you started.
Help with managing your condition. PANZYGA offers a variety of downloadable resources to help you throughout treatment, including guides to keep track of your therapy, and more.
All in one place. Pfizer IGuide™ can help you understand your insurance coverage and out-of-pocket costs for your prescribed PANZYGA, as well as identify financial assistance options for which you may be eligible.
Learn more here. A free mobile app designed to help support patients throughout their IVIg treatment experience.
Sign up for additional information to help you understand more about PANZYGA and to receive free resources.
Learn more here. With this program, eligible patients may pay as little as $0 for PANZYGA.*
- Patients must have commercial insurance to be eligible
- Patients are not eligible if they are enrolled in a state or federally funded insurance program
*The PANZYGA Co-Pay Program provides eligible patients assistance of up to $12,500 per year or the cost of a patient’s co-pay in a 12-month period (whichever is less). The PANZYGA Co-Pay Program is good only in the U.S. and Puerto Rico. No membership fees. Federal and state health care beneficiaries are not eligible. Private insurance only. Eligibility and restrictions apply. See Terms and Conditions at http://pfizeriguide.com






